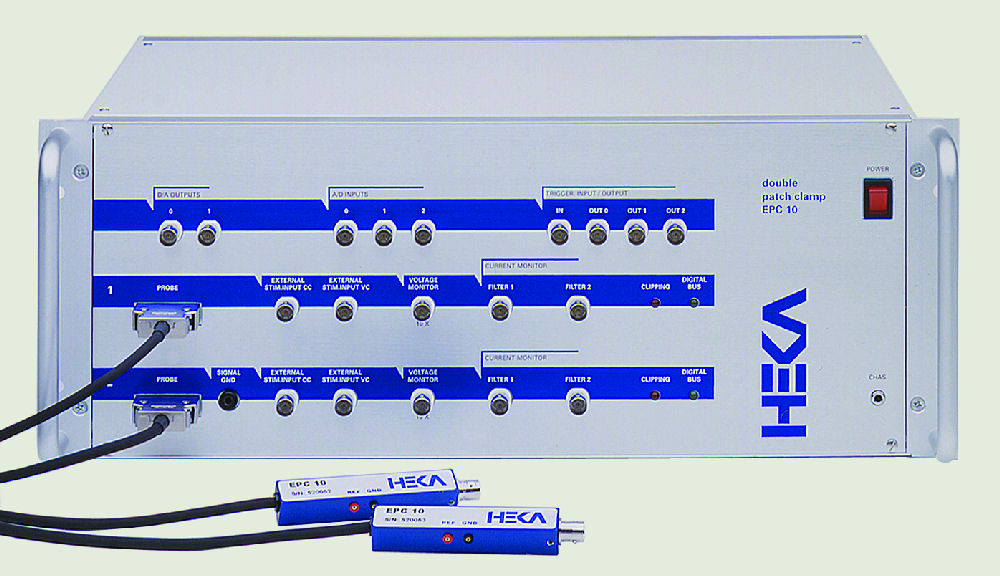
Continuing the tradition of providing the world’s best electrophysiology amplifiers, the EPC 10 USB Double and Triple patch clamp amplifiers are the optimal instruments for performing double or triple patch experiments. Although either two (EPC 10 USB Double) or three (EPC 10 USB Triple) amplifiers are combined in a single housing; each amplifier is completely independent with clearly defined operation and handling. HEKA’s software stimulates the desired amplifier and selected channels are programmed without tedious connection of cables by the user.
The amplifiers and headstages are clearly identified, thus, the user can immediately assign the amplifiers to particular patched cells. Although independent, the amplifiers can be stimulated simultaneously with resulting simultaneous data acquisition. Current or voltage signals from multiple amplifiers can be recorded, displayed and even analyzed online. This versatility makes these amplifiers ideal instruments for experiments such as studying gap junction potentials, pre- and post-synaptic events, and usage in amperometric measurements.
The versatility of the EPC 10 USB Double and EPC 10 USB Triple stem from their adjustment and control at the software level. Leak currents, capacitances, and series resistances can instantly be neutralized automatically while you still retain the possibility of full manual control of the amplifier. Voltage- and Current-Clamp experimental protocols are easily automated; not only ensuring simplicity, great speed and accuracy but also providing enormous flexibility for the integration of extensions such as photometric and fluorescence measurements.
The EPC 10 USB Double and EPC 10 USB Triple also provide economical solutions in comparison with the combination of several individual instruments. They also have the advantages of optimized noise performance and grounding over that of multiple external amplifiers.
In current clamp (CClamp) mode, the membrane potential (Vm) is measured and used to compute a current waveform which is fed back into the cell. As a result, slow voltage drift and other artificial voltage-dependent conductances are controlled and adjusted to a fixed value. This negates any readjustment of the holding current while conducting CClamp experiments.
In CClamp mode, the headstage acts as a voltage follower circuit, which guarantees very fast and accurate membrane potential recordings ("True CClamp"). Similar to a classical microelectrode amplifier, the cell voltage is measured on a very high input resistance.
The slim shape of the headstage allows optimal access to your cells. The connection to the pipette holder is achieved via a stable BNC connector.
Adapter plates for various micromanipulators are available from HEKA.
Features:
- Full computer control (Mac and Windows based)
- Can be used with PatchMaster on Windows and Mac, and with PatchMaster Pro and TIDA on Windows.
- EPC DLL (dynamic link library) is available to control the amplifier from your own applications (Windows)
- Automatic self-test and calibration
- Automatic Capacitance neutralization
- Capacitance tracking
- Automatic leak subtraction
- Optimized, built-in interface
- Integration with the LIH 8+8 AD/DA interface with isolated USB 2.0 connection to the host computer
- ultra slim-line headstage design with improved noise performance
- True Current Clamp mode
- Low Frequency Voltage Clamp (LFVC)
- Gentle Switch option to CC mode (injection current is equal to Imon in VC)
- 16 Digital outputs
- integration of hardware and software eliminates compatibility problems on Windows operating systems.
- True noise measurements from 100 Hz to 15 kHz
- Built in sound capabilities
- Can be extended with an additional LIH 8+8
- Digital I/O connector for EPC 8 or TIB 14S
- Resistor switching headstage with three gain ranges that can be switched during the experiment
|
|

